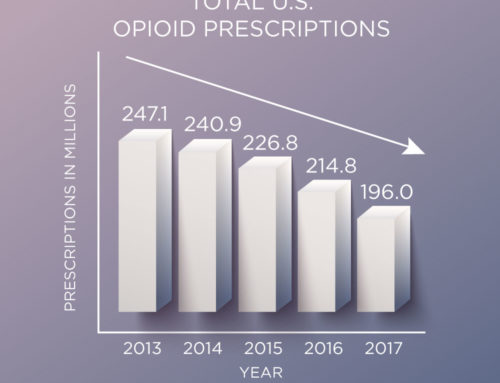
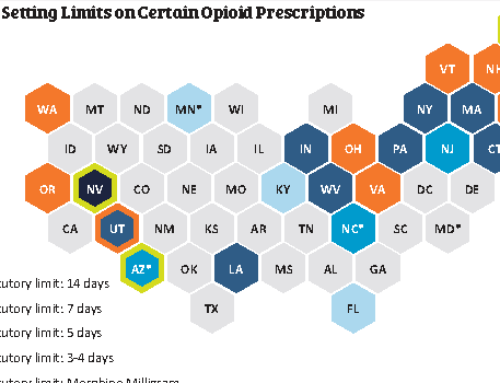
Two powerful tools to help us all combat the growing Opioid Crisis: the FDA’s new Opioid Analgesic Risk Evaluation and Mitigation Strategy (REMS) and Congress’s SUPPORT for Patients and Communities Act.
FDA REMS
In September, the FDA approved a new Opioid Analgesic Risk Evaluation and Mitigation Strategy (REMS) that expands upon the previous (2012) Opioid Analgesics REMS. The new REMS requires training also be made available on immediate-release opioid analgesics and offered to prescribers and nonprescribers alike who are involved in the management of patients with pain. In tandem, the FDA also approved the new Education Blueprint for Health Care Providers Involved in the Treatment and Monitoring of Patients with Pain (Blueprint). The updated Blueprint calls for CE/CME to incorporate the new REMS content, as well as a new, short primer on addiction medicine.
The new REMS and Blueprint reflect the FDA’s belief that more health care providers should be educated about the safe use of opioids. In addition to prescribers (which could include physicians, nurse practitioners, physician assistants, and dentists), non-prescribing nurses and pharmacists are now also targeted for education. The Blueprint specifically mentions “all healthcare providers who participate in the treatment and monitoring of patients who receive opioid analgesics”. Whether or not they write or dispense a prescription, these providers can help ensure proper use and appropriate clinical oversight.
SUPPORT Act
On October 24, 2018, President Donald Trump signed into law bipartisan legislation to combat the opioid crisis. The legislation is called The Substance Use-Disorder Prevention that Promotes Opioid Recovery and Treatment (SUPPORT) for Patients and Communities Act.


Energy and Commerce Committee Chairman Greg Walden (R-OR) and Health Subcommittee Chairman Michael C. Burgess, MD (R-TX) issued a statement citing:
“This landmark legislation is the culmination of months of bipartisan work to combat the opioid crisis from every angle. H.R. 6 brings critical support to the communities most desperately in need, provides new tools and resources for those on the front lines of this crisis, and helps stop the flow of deadly drugs across our borders. Rarely can we say that legislation will save lives, but there is no doubt that H.R. 6 will do just that.”
Taken together with other recent legislation and appropriations (the Comprehensive Addiction and Recovery Act, 21st Century Cures Act, $4 billion appropriated in the 2018 omnibus, and $6.7 billion in the recent Defense-Labor-HHS appropriations package), this is the most significant congressional effort against a single drug crisis in history.
The SUPPORT Act’s key tactics fall into four categories:
Treatment and Recovery
- Improve and expand access to treatment and recovery services
- Provide incentives for enhanced care, coordination, and innovation
- Establish comprehensive opioid recovery centers
Prevention
- Encourage non-addictive opioid alternatives to treat pain
- Improve data to identify and help at-risk patients and families
- Address high prescribing rates while enhancing prescription drug monitoring programs
Protecting Communities
- Give law enforcement tools to remove dangerous drugs from our communities
- Better intercept illicit opioids at international mail facilities
- Improve access to federal resources for local communities
Fighting Fentanyl
- Better tackle ever-changing synthetic drugs
- Crack down on foreign shipments of illicit drugs
- Provide grants for local communities to combat fentanyl
For more information on H.R. 6, see this 1-minute highlights video, a 1-page overview, a section-by-section summary, and the text of legislation.
Get ready! Take a CO*RE safe opioid prescribing course, available online, or access practical tools here.