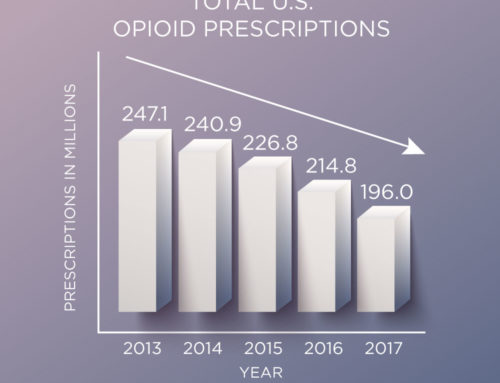
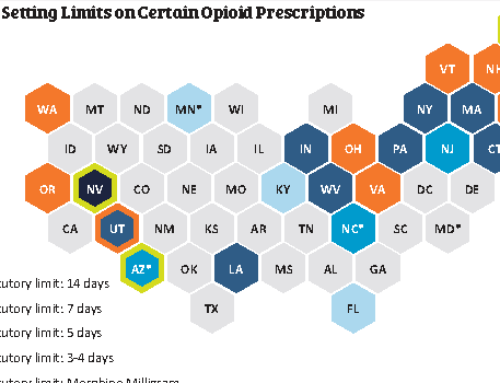
In November 2017, the FDA released a report with analysis and recommendations for the use of continuing education (CE/CME) for Risk Evaluation and Mitigation Strategy (REMS). But what is a REMS? And how is healthcare provider CE/CME related to it? This new FDA Feasibility Report addresses these questions.
A Risk Evaluation and Mitigation Strategy (REMS) is a required risk management strategy, authorized by the FDA, for certain drugs to ensure that the benefits of the drug outweigh its risks. Historically, REMS have included components such as a Medication Guide, a patient package insert, a communication plan, and/or elements to assure safe use (ETASU) such as training for health care providers (HCPs), requiring that patients using the drug be monitored, or that the drug be dispensed to patients with evidence of safe-use conditions. The ER/LA Opioid Analgesics REMS is the first to require that the ETASU training for HCPs is offered by accredited CE/CME providers according to an FDA-developed Blueprint, as is occurring with CO*RE.

The REMS authority has enabled FDA to approve products with serious risks that might otherwise not have been made available to patients. However, the time and resources needed to comply with some REMS has led to concerns about burden on the healthcare system, which may ultimately limit patient access to drugs. In 2011, FDA launched the REMS Integration Initiative with the goal of standardizing and better integrating REMS into the healthcare system. This initiative resulted in many guidances and reports, including the current one examining feasibility of using CE/CME credit for REMS-required HCP education.
The November 2017 FDA Feasibility Report details how the FDA solicited feedback from multiple stakeholders, reviewed pertinent literature, considered results from an exercise conducted by the CE accrediting bodies, and considered the lessons learned from the CE training program for the ER/LA Opioid Analgesics REMS. CO*RE partners provided input to the process as stakeholders as well as sharing our overall experiences, outcomes, and publications including our learner survey results published in the journal Substance Abuse.

Some key recommendations from the FDA report include:
- CE/CME could be developed as part of a REMS following initial drug approval but not at the time of initial drug approval
- An FDA-developed Blueprint will help ensure such CE/CME materials place necessary emphasis on risk messages REMS requirements
- Stakeholders should address how risk messages and REMS requirements in CE/CME materials will remain current if new safety information becomes available during the drug’s lifecycle
- If CE/CME activities occur in the context of a broader educational review of a topic, the key REMS-related risk information must be readily accessible to HCPs because not all learners will need the broader content
- Alignment of standardized assessment methods for both the CE/CME activity and REMS aspect
- Particularly if training is a prerequisite to prescribing, the education must be readily accessible on a continuous basis to allow timely completion by HCPs, and the functionality must connect completion to prescribing/dispensing authorization
By actively contributing to and participating in these ongoing developments, CO*RE ensures our offerings meet the mutual goals of stakeholders while helping pave the way to improvements that align with our mission of promoting individual and population health and public safety.
********************
Learn about safe opioid prescribing in a CO*RE course, available live or online, or access practical tools here.
For Further Reading
- REMS and Continuing Education for Health Care Providers: FDA Feasibility Report
- ER/LA Opioid Analgesics REMS (full document)
- REMS Integration Initiative
- FDA Is Making It Easier To Meet Required Extra Safety Measures for Certain FDA-Approved Drugs
- ER/LA Opioid Analgesics REMS (website)
- FDA Blueprint for Prescriber Education for Extended-Release and Long-Acting Opioid Analgesics (“FDA Blueprint”)
- ER/LA opioid REMS and accredited education: Survey results provide insight into clinical roles, educational needs, and learner preferences